Covered stents, stent-grafts, and valved frames are at the center of today’s most advanced interventional and structural heart therapies. These devices pair precise metallic scaffolds with the performance of engineered polymer coverings, to repair, reinforce, or replace vessels and ducts in high-risk cardiovascular, endovascular, and non-vascular procedures. From EVAR and TEVAR systems to tricuspid valves, PFO occluders, and intracardiac shunts, covered stents and frames are redefining what’s possible in minimally invasive care.
Where They’re Used
Cardiovascular and Structural Heart:
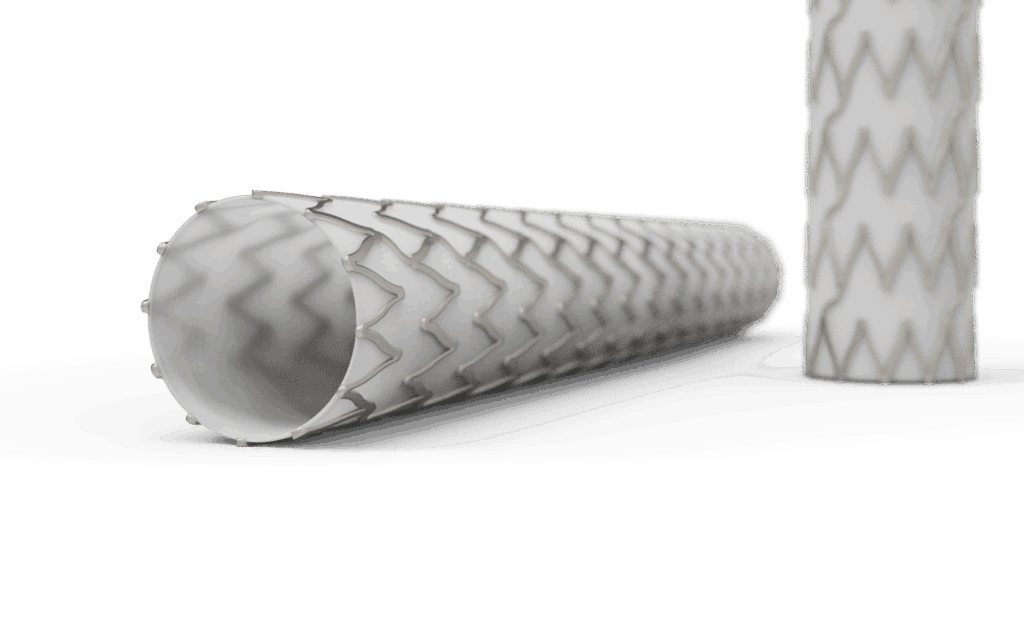
Covered stents and frames are fundamental in transcatheter valve systems (TAVR, TMVR, and TTVR), where nitinol or cobalt-chromium frames support bioprosthetic leaflets and sealing skirts to prevent paravalvular leak. Valved stents, occluders, and shunt systems, including LAA (Left Atrial Appendage) and PFO (Patent Foramen Ovale) occluders as well as interatrial shunts designed to regulate cardiac pressure, use ePTFE or polyester membranes to achieve durable sealing and controlled flow while staying flexible for catheter delivery.
Aortic and Peripheral Vascular:
In endovascular aneurysm repair (EVAR) and thoracic EVAR (TEVAR), covered stent-grafts composed of polyester or ePTFE are deployed to exclude aneurysmal sacs and dissections, reline damaged vessels, and restore hemodynamic stability. Many incorporate catheter-based anchoring and docking systems, including barbs, flares, or flanged nitinol elements, to ensure stable fixation and minimize migration under pulsatile loads. Peripheral covered stents are also used to treat iliac, femoral, and renal artery disease, offering rapid vessel sealing and lowering restenosis risk.
Neurovascular:
Ultra-thin covered stents are emerging for intracranial aneurysm exclusion and vessel reconstruction. ePTFE membranes laser-bonded to micro-patterned nitinol scaffolds allow selective flow diversion while maintaining flexibility for tortuous anatomy.
Airway, Gastrointestinal, and Biliary:
In non-vascular indications, self-expanding metal stents (SEMS) with silicone or polyurethane coatings maintain patency in the trachea, bronchi, esophagus, or biliary ducts while preventing tissue ingrowth. These coatings are biostable, smooth, and lubricious, reducing biofouling and facilitating atraumatic removal or repositioning.
Urologic and Shunt Systems:
Covered stents and valves are also applied in ureteral, prostatic, and cerebrospinal shunt systems, where low-durometer polyurethane and silicone layers deliver durability and controlled compliance. Heart and vascular shunts, particularly interatrial flow-regulating implants, often integrate bioresorbable or semi-permeable membranes to manage pressure gradients between chambers.
Advanced Materials for Complex Requirements
Performance depends on the interaction between the metal frame and the chosen polymer covering. Medical Murray and its partners work with a diverse range of materials, each optimized for specific end-use performance. We routinely work with:
- Expanded PTFE (ePTFE): Microporous, inert, and non-thrombogenic, ideal for vascular sealing and controlled permeability.
- Polyester (PET): Strong, suture-retentive, and dimensionally stable, commonly used for large-diameter grafts.
- Polyurethane (PU): Excellent fatigue resistance and elongation for dynamic lumens such as ureters and bronchi.
- Silicone: Smooth, flexible, and biostable, well suited for airway, GI, and shunt applications.
- Bioresorbable polymers (PLLA, PGA, and PCL blends): Emerging materials for temporary scaffolds and covered stents that gradually resorb, leaving behind a natural lumen without permanent implant material.
Hybrid coverings are increasingly common, combining ePTFE, polyester, and/or polyurethane layers to balance low profile, sealing performance, and promotion of tissue ingrowth for long-term fixation and healing.
How We Join and Encapsulate
Getting polymer coverings to reliably bond to metal frames requires tight process control. Medical Murray employs a full range of joining and encapsulation technologies tailored to both vascular and non-vascular applications:
- Thermal Lamination and Encapsulation: Controlled heat and pressure fuse polymer films over the stent frame to achieve seamless coverage. Tie-layer materials are often used for ePTFE encapsulation, improving adhesion and minimizing delamination.
- Sophisticated Tooling and Fixtures: Precision-designed mandrels and compression tooling achive wrinkle-free encapsulation, concentric wall thickness, and accurate bonding along complex geometries.
- Heat Shrink Bonding: Dual-layer polymer assemblies are consolidated over the frame with controlled heat cycles to achieve uniform encapsulation.
- Adhesive Bonding: Medical-grade silicone and urethane adhesives are used where compliance and localized flexibility are required.
- Laser Micro-Texturing: Surface modification of nitinol or stainless steel enhances polymer-to-metal bonding.
- Micro-Sewing and Suturing: For large-diameter stent-grafts or occluders, polyester graft material is hand-sewn or machine-stitched to the frame using USP 7-0 to 5-0 sutures for uniform strength and flexibility.
- Overmolding and Dip Coating: Silicone or polyurethane coatings are applied through precision-controlled dip or spray processes, yielding pinhole-free barriers.
All processes undergo adhesion, burst, leak, and fatigue testing, ensuring reliability under physiological conditions.

Where Work Happens
Medical Murray’s vertically integrated operations span laser cutting, nitinol shape setting, ePTFE encapsulation, polymer lamination, sewing, and cleanroom assembly under ISO 13485 quality systems. Production and testing occur in Class 7 cleanrooms with full traceability to implant-grade materials.
Our North Carolina facility, now operated by Aptyx, a global full-service provider to the MedTech industry, leads in ePTFE encapsulation, lamination, and precision sewing/suturing for stents and frames. With end-to-end capabilities, the site delivers ultra-thin, fatigue-resistant coverings for complex transcatheter implants, valve frames, and occlusion devices.
Engineering the Future of Minimally Invasive Implants
The boundaries between stents, frames, valves, and occluders are rapidly blurring. Hybrid devices now combine structural support, flow regulation, and biological integration in a single implant. Advances in catheter-based anchoring, docking, and valve actuation are enabling treatments that were once only possible through open surgery.
From EVAR and TEVAR stent-grafts to transcatheter tricuspid and mitral valves, LAA/PFO occluders, heart shunts, and biointegrative airway or GI SEMS, the engineering complexity continues to grow, requiring precise alignment of materials, mechanics, and manufacturing.
At Medical Murray, we partner with innovators to design, prototype, and scale these life-saving technologies. Our expertise in metal processing, polymer science, and catheter integration bridges early-stage development through commercial-scale manufacturing, helping bring safer, more effective implants to patients worldwide.
