Embolic filters protect patients from harmful debris (emboli) that can travel through the bloodstream during or after medical procedures. These devices are designed to capture clots, plaque, or calcific particles before they can reach critical organs such as the brain, heart, or lungs.
Types of Embolic Filters
| Single-Use Filters | Implantable Filters |
| Use: Temporary use during procedures like TAVR (transcatheter aortic valve replacement) or peripheral vascular intervention. Removed at the end of a case. | Use: Long-term use, such as IVC (inferior vena cava) filters, for patients with ongoing risk of embolization. |
| Design Priorities: low profile, deliverability, safe retrieval. | Design Priorities: durability, fracture resistance, long-term flow compatibility. |
Though the end goal is always embolic protection, the design challenges differ significantly between single-use and implanted filters. Below we’ll explore the requirements and considerations that guide development in both markets.
Design Priorities for Single-Use Filters
Temporary filters are used in procedures where the risk of embolization is high but time is limited.
- TAVR: Filters placed in the aorta, carotid or brachiocephalic arteries reduce the risk of stroke caused by debris released when the diseased valve is replaced. The challenge here is creating a low-profile, highly reliable system that can reach complex anatomy and then retrieve smoothly.
- Peripheral Vascular Interventions: Procedures such as atherectomy or thrombectomy may release debris downstream. Filters must expand to cover large vessel diameters, hold significant embolic load, and collapse safely for removal.
In these cases, design priorities focus on deliverability, embolic capture efficiency, and safe retrieval, all within the tight window of a single procedure.
Design Priorities for Implantable Filters
Implantable filters are used when patients face sustained risk of embolization.
- IVC filters: Implanted in the inferior vena cava to prevent clots from the lower body reaching the lungs. Some are permanent, while others are designed for retrieval after several weeks or months.
Implantable filters require a very different focus than temporary filters. They must remain in the body long term, resist fracture, avoid migration, and maintain blood flow without causing thrombosis. Retrievable versions must balance stability with the ability to collapse safely when it’s time for removal.
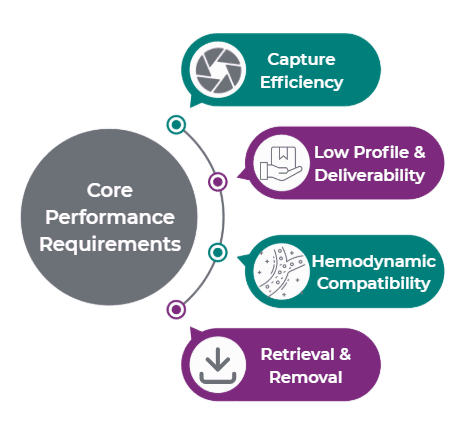
Core Performance Requirements
Across both markets, there are four primary performance requirements:
Capture Efficiency
Filters must balance pore size and geometry to catch harmful particles while still allowing smooth blood flow.
- Particle size: Pores must be small enough to trap emboli (>100 µm typically) but not so small that they restrict flow.
- Geometry: Uniform pore distribution improves capture but adds manufacturing complexity.
- Coverage: Proper wall apposition to make sure no emboli slip past the filter.
Low Profile & Deliverability
Ease of delivery is a major adoption factor.
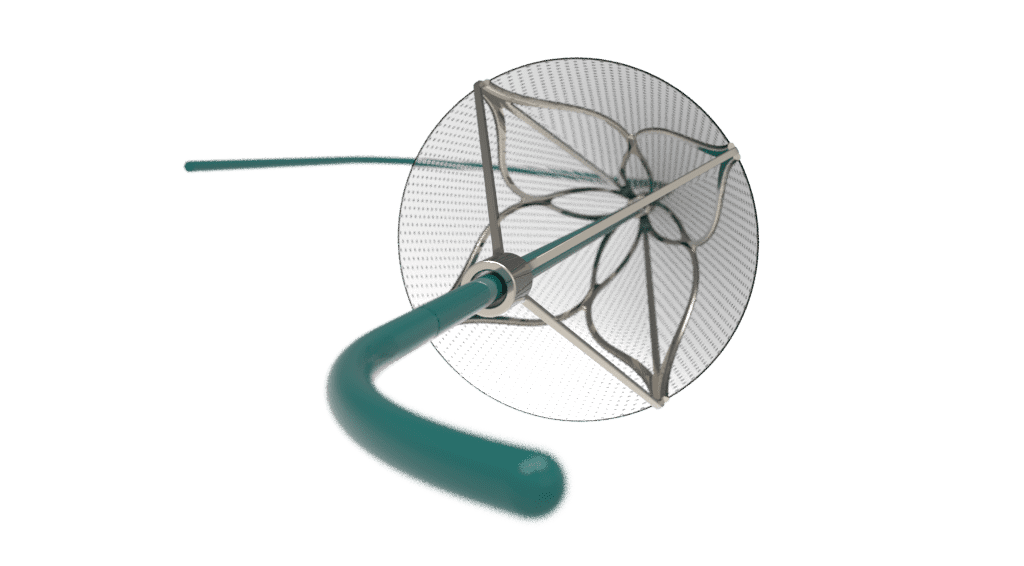
- Cross tortuous vasculature with minimal vessel trauma.
- Catheter shaft design (stiffness gradients, coatings) supports trackability.
Hemodynamic Compatibility
Filters should capture debris without disrupting natural blood flow.
- Resistance to thrombosis and hemolysis is critical.
- Pore density, strut thickness, and materials affect flow dynamics.
- Computational fluid dynamics (CFD) helps optimize pore geometry and reduce flow disruption.
Retrieval & Removal
Single-use filters must be removed easily, and implanted filters must offer safe retrieval when indicated.
- Filters should collapse and re-sheath reliably, even after long deployment.
- Retrieval forces must be low to avoid vessel trauma.
- Designs must hold embolic load without compromising retrieval.
Engineering & Design Considerations
Material Selection:
- Metallic frameworks (e.g., nitinol) provide elasticity, self-expansion, and fatigue resistance.
- Polymer meshes (ePTFE, polyurethane, bioresorbable) for porosity & thrombogenicity reduction.
- Hybrid designs for strength + compatibility.
Deployment Mechanism:
- Self-expanding nitinol baskets are widely used and require precise thermal setting and cutting.
- Balloon-expandable for controlled placement.
- Collapsible frames must reliably re-sheath for removal.
Vessel Wall Interaction:
- Adequate radial force for anchoring.
- Tapered, atraumatic tips reduce vessel damage.
Radiopacity & Visualization:
- Platinum-iridium or tantalum markers for fluoroscopic visibility.
- Marker placement must enable visualization during both deployment and retrieval.
Fatigue & Durability:
- Implanted devices are exposed to millions of cardiac cycles, requiring strong fatigue resistance.
- Verified via finite element analysis (FEA) + accelerated wear testing.
Manufacturing Considerations
Building embolic filters requires precision and careful handling of delicate geometries.
- Laser cutting & shape setting: Key for nitinol frameworks, demanding precise tolerances.
- Polymer processing: Techniques such as dip molding, spray coating, electrospinning, laser drilling, or perforation create controlled porosity.
- Assembly & bonding: Combining metals and polymers requires adhesives, thermal bonding, overmolding, or sintering.
- Sterilization & packaging: Processes must preserve pore structure and material integrity while protecting the device during shipment.
Testing & Validation
Testing reflects whether a filter is intended for single-use or implantation.
- Single-use: capture efficiency, embolic load capacity, retrieval forces, deliverability.
- Implantable: long-term fatigue, fracture resistance, vessel wall safety, retrieval reliability. Specific standards like ISO 25539 (vascular implants).
Both must meet standards like ISO 10993 (biocompatibility), along with FDA expectations.
The Future of Embolic Filters
The next wave of embolic filters is moving toward:
- Bioresorbable filters that dissolve after use.
- Drug-eluting surfaces to reduce thrombus formation.
- Smart filters that incorporate sensors for real-time monitoring.
These innovations are driven by the need for greater safety, easier use, and integration with advanced endovascular therapies. Design requirements might vary, but the common goal remains the same: protect patients from life-threatening emboli while the device is safe for patients and reliable for physicians. At Medical Murray, we partner with innovators to design, develop, and manufacture embolic filters that meet these demanding requirements. Contact us to discuss your project!
