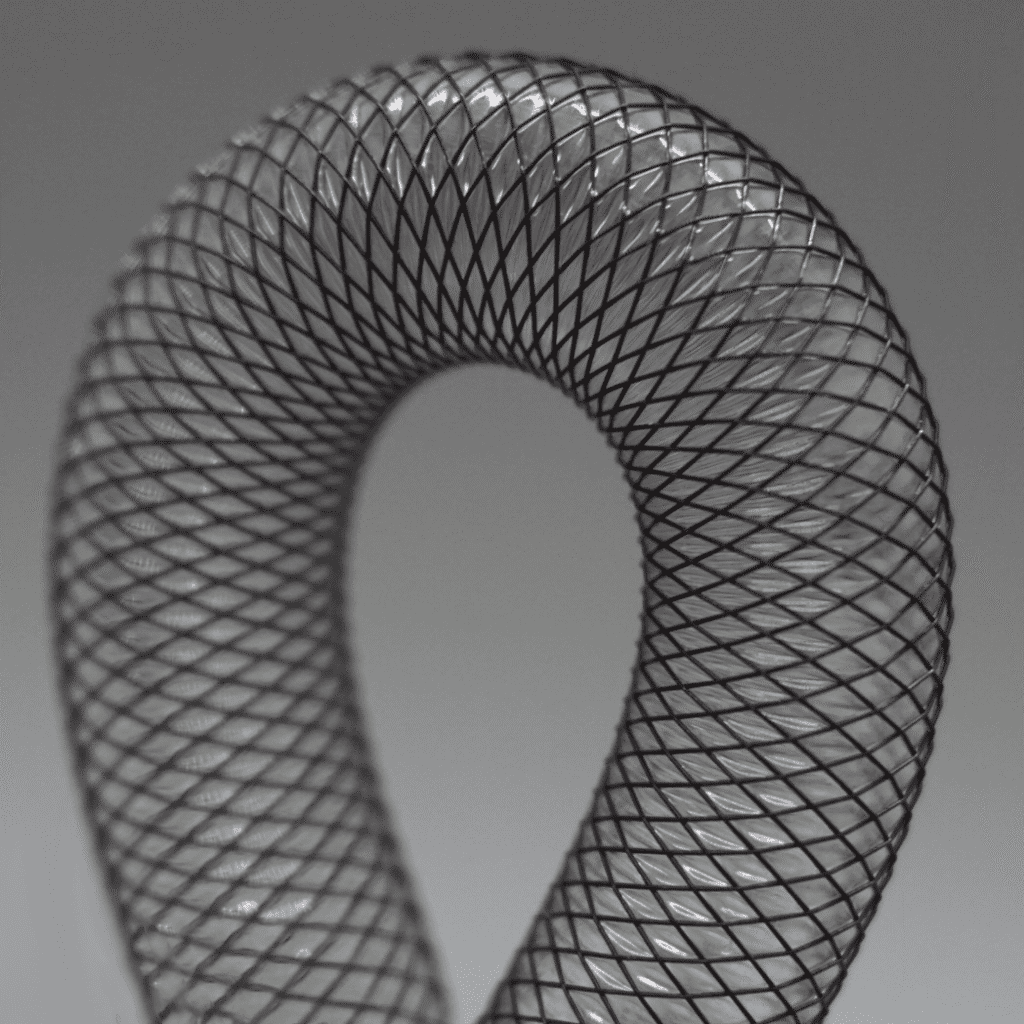

Braiding is a critical process for manufacturing medical devices, particularly in the production of catheter shafts, stents, retrieval devices, and embolic filters. Braiding involves intertwining multiple strands resulting in a robust structure with unique attributes including torqueability, flexibility, and kink-resistance.
Braided devices can also be made in more complex shapes using custom mandrels and fixtures. This makes braided materials a great fit for custom applications and more specialized devices.
Braids can also be engineered to expand when compressed or unsheathed, which can be useful in certain medical device applications. The ability of a braid to expand when compressed is primarily influenced by the braid’s density or pick count. A lower pick count allows for more radial expansion when the braid is compressed. Expert manipulation of these factors is essential when creating self-expanding stents and similar medical devices.
Key specifications in the medical device braiding process include:
- Material Selection: The choice of material directly impacts the performance characteristics of the braid. Nitinol and stainless steel are commonly used for braids. Polymers such as nylon, Polyether ether ketone (PEEK), Liquid Crystalline Polymer (LCP), and bioabsorbables are also in demand. Each offers unique properties like strength, flexibility, biocompatibility, and radio-opacity that must match the performance requirements for the particular device.
- Wire Diameter or Filament Size and Shape: The diameter of the wire, filament, or ribbon used in the braid can influence the strength, flexibility, and profile of the device.
- Braid Pattern: The pattern or weave of the braid (Half Load (1O1U1), Full Load (1O2U2), Diamond(2O2U2), etc.) can influence the kink resistance, torqueability, flexibility, expandability, and pushability of the device.
- Braid Density: The density of the braid can be modified to achieve different mechanical properties. For instance, a higher pick count enhances torque transmission, while a lower pick count improves flexibility and allows for radial expansion. For catheter shaft reinforcements, the density affects the kink resistance, stiffness/flexibility, and pushability.
- Length and Diameter of the Device: These parameters are determined by the anatomical requirements of the device’s intended application.
- End Configuration: The device may need different end configurations depending on the application. Closed/looped end braids allow for an atraumatic braid end, which is essential for applications where exposed braid may become caught on tissue and cause damage.
- Covering: If the braided device requires a covering to create a sealed, continuous surface that ensures fluids pass through the stent graft. Potential braid coverings commonly include compliant materials such as silicone or polyurethane.
- Variable Diameter and Complex Geometries: With the use of custom mandrels or core pins, braids with variable diameters and more complex geometries can be achieved, enabling us to cater to unique and challenging device designs.


These specifications inform the medical braid manufacturing process at Medical Murray, where we specialize in catheter shafts, stents, retrieval devices, including clot retrieval devices, and embolic filters. We leverage a wide range of materials, and our specialized equipment helps achieve precise and consistent braiding for specific patterns.
Whether you’re still considering the design of your braid or you’re ready for full-scale manufacturing, Medical Murray is here to help you through each step to ensure your device can start improving patient care as soon as possible.
By Tim Spiekerman and Tanner Hargens
