Blog
Scaling Catheter Manufacturing: From Prototype to Pilot to Full-Scale Production
Scaling a catheter program is not simply about increasing production volume. It is about increasing maturity across four critical areas: device design, manufacturing process, quality system, and cost structure. Most catheter programs move through three distinct phases: Each phase serves a distinct purpose. When expectations are aligned with that purpose, catheter scale-up becomes predictable, controlled,…
Read MoreWhy Women’s Health and FemTech Medical Devices Need More Than Great Ideas
Women’s health has been underrepresented in medtech for far too long.Not because the clinical needs are small, but because they have often been treated as niche. We do not see it that way. At Medical Murray, women’s health is not a side category or a temporary focus. It is a critical and growing area of…
Read MoreWhat It Takes to Develop Pulse Field Ablation (PFA) Catheters with Manufacturing in Mind
Pulse field ablation (PFA) is rapidly transforming electrophysiology (EP). Its non-thermal mechanism, tissue selectivity, and reduced collateral damage have accelerated interest in next-generation PFA mapping and ablation catheters. At the same time, many EP catheter development programs stall before commercialization. Funding constraints, underestimated technical complexity, and late-stage manufacturing surprises are common. While full-scale PFA production…
Read MoreIVUS vs. OCT Catheters: What It Takes to Hybridize Modalities
IVUS and OCT each offer something valuable in intravascular imaging. IVUS gives deeper visualization through blood and plaque, while OCT provides very fine detail close to the vessel wall. For years, companies have treated them as separate tools because the engineering needs behind each system are so different. Now more teams are trying to bring…
Read MoreWhat Happens After Capture?
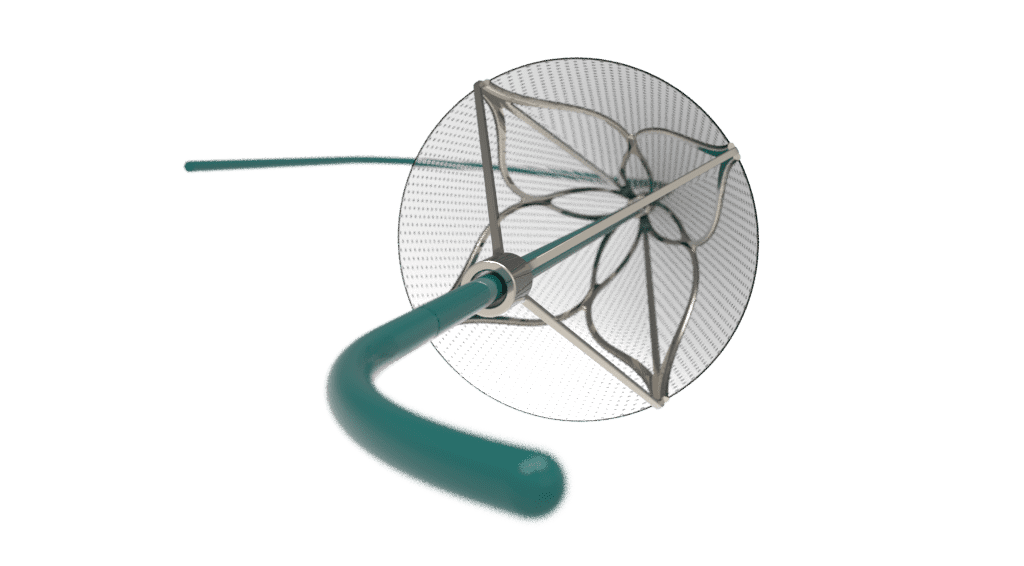
Embolic filters protect downstream vessels while thrombectomy devices actively remove clots, but both face the same engineering challenge: capturing debris is only step one. The true test of a device is what happens after the material is captured. What happens after material is captured ultimately determines whether a device is safe, effective, and clinically successful.…
Read MoreAdvanced Braiding Techniques for Interventional Devices
Braiding is a core technique in many interventional medical devices. Most people know braiding from its role in reinforcing catheter shafts, but it also plays a critical role in devices like thrombectomy tools, embolic filters, septal occluders, left atrial appendage (LAA) implants, and even some stents. The braid pattern and material choice have a big…
Read MoreManufacturing Spotlight: Covered Stents and Frames for High Impact Procedures
Covered stents, stent-grafts, and valved frames are at the center of today’s most advanced interventional and structural heart therapies. These devices pair precise metallic scaffolds with the performance of engineered polymer coverings, to repair, reinforce, or replace vessels and ducts in high-risk cardiovascular, endovascular, and non-vascular procedures. From EVAR and TEVAR systems to tricuspid valves,…
Read MoreThe Future of FemTech: Implantables, Catheters, & Delivery Systems
Women’s health is finally getting the innovation it deserves, with investment shifting from apps and wellness to advanced medical devices that solve long-standing clinical problems. For companies experienced in catheters, implants, and interventional systems, the next generation of women’s health devices will rely on material science, manufacturing control, and miniaturized mechanics to make care safer,…
Read MoreWhy More Medtech Companies Are Manufacturing in Illinois
California has long been a hub of medtech innovation. Many of the world’s best ideas, technologies, and companies were born there. But as organizations move from R&D to large-scale manufacturing, many are expanding beyond California, and Illinois has emerged as a compelling destination. 1. Cost Advantages 2. Logistics & Distribution 3. Skilled Workforce 4. Central…
Read MoreDesign Considerations for Embolic Filters
Embolic filters protect patients from harmful debris (emboli) that can travel through the bloodstream during or after medical procedures. These devices are designed to capture clots, plaque, or calcific particles before they can reach critical organs such as the brain, heart, or lungs. Types of Embolic Filters Single-Use Filters Implantable Filters Use: Temporary use during…
Read More