Blog
Choosing the Right Polymers for Soft Tissue Implants: ePTFE, PET, TPU, Bioabsorbable, and More
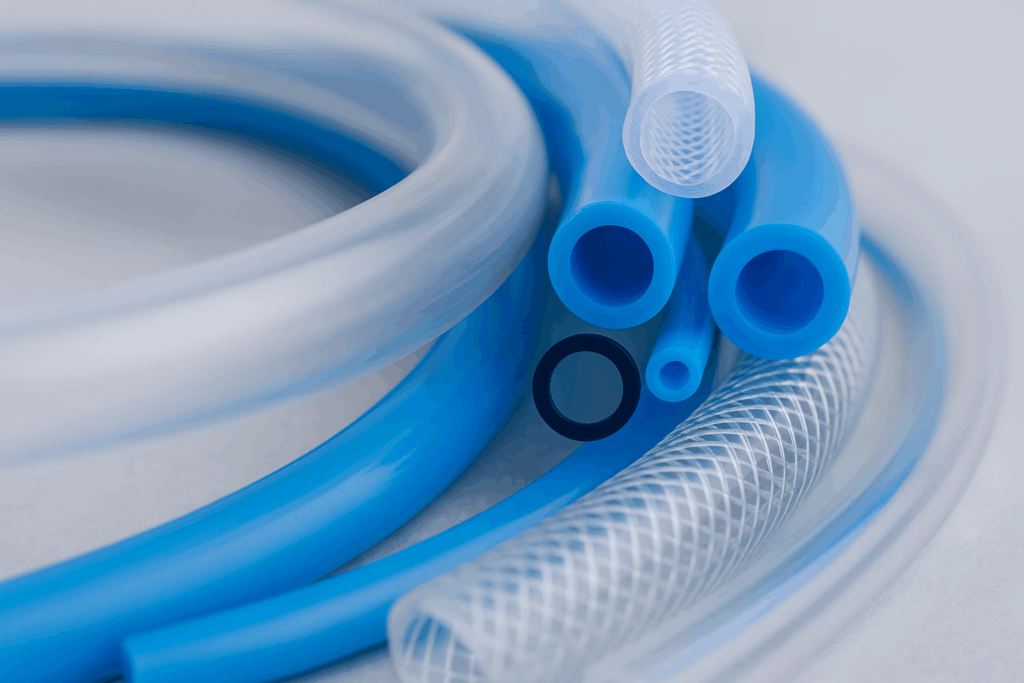
When developing implantable medical devices for soft tissue applications (vascular, gastrointestinal, urology, and more), one of the earliest and most critical decisions is selecting the right polymer material. The polymer you choose directly impacts biocompatibility, mechanical performance, and even regulatory approval timelines. And in many designs, the polymer needs to work in tandem with metallic…
Read MoreWhy Integrated Medical Device Testing Leads to Better Design
When you’re developing a complex medical device, especially a Class III product, testing should be part of the process from the very beginning, not something you scramble to do at the end. At Medical Murray, we believe good testing isn’t just about passing requirements. It’s about building a better, safer, and more reliable product that’s…
Read MoreFrom Idea to DHF: Building a Regulatory-Ready Medical Device
Startups love prototypes. They’re tangible, testable, and exciting. But if you want to commercialize a Class II or Class III medical device, the physical product is only half the story. The other half? Telling the FDA why the device is safe and effective. That story is captured in your Design History File (DHF), the structured…
Read MoreBringing Smart Interventional Medical Devices to Life
Integrating Electronics into Catheters and Implants Smart medical devices are reshaping interventional healthcare. Catheters and implantable devices are now expected to do more including integrate sensors, collect data, enable robotic-assisted surgery, and respond in real time. At Medical Murray, we integrate electronics into complex catheters and implantable medical devices, supporting the development of Class II…
Read MoreCleanroom Design for Complex Catheter Assembly
Building complex catheters takes more than just clean space. These are often delicate devices that can be long, flexible, and sometimes awkward to handle. The environment where they’re assembled must support that kind of work. At Medical Murray, we’ve designed our cleanrooms to meet the unique demands of catheter and implant manufacturing. That includes ergonomic…
Read MoreHow We Support FDA Readiness for Class III Medical Devices
What we do to help our partners get through testing, quality, and documentation Bringing a Class III medical device to market takes more than a good design. It takes a clear process, strong documentation, and the right testing to meet FDA requirements. At Medical Murray, we’ve built our team, tools, and systems specifically to support…
Read MoreWhy Radiopacity Matters in Next-gen Medical Devices
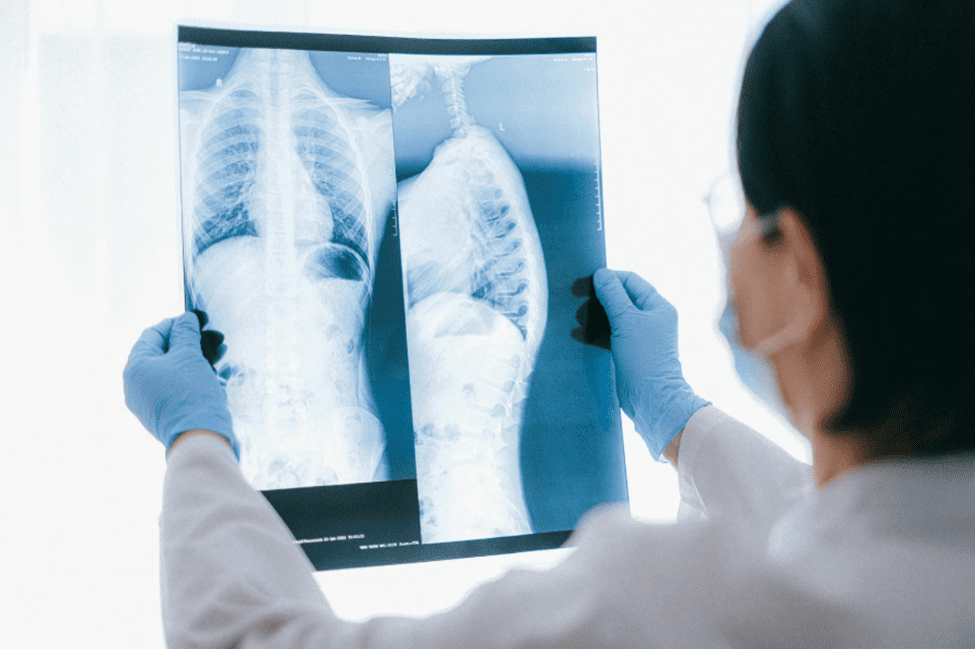
When it comes to minimally invasive procedures, being able to see the device matters. Whether a doctor is guiding a catheter or placing an implant, they need to rely on imaging to know the exact location of the device. That’s where radiopacity comes in. At Medical Murray, we develop and manufacture advanced radiopaque catheters and…
Read MoreWhat It Takes to Manufacture Pediatric Catheters
Medical Murray | Complex Catheter and Implant Solutions Manufacturing pediatric catheters isn’t just about shrinking things down. It’s about rethinking the design, materials, and entire manufacturing process to work safely and effectively in smaller, more sensitive anatomy. At Medical Murray, we’ve worked on a wide range of pediatric medical devices, from neonatal vascular access to…
Read MorePractical Advice for Medical Device Startups
A polished slide deck can open doors and land seed money. But Series A investors, and eventually clinicians and patients, pay for working products. This means devices that can be built, sterilized, shipped, and cleared by regulators. Use this reality-check to move from seed funding to first commercial sales without running out of runway. A Pretty…
Read MoreLaser Welding in Medical Device Manufacturing
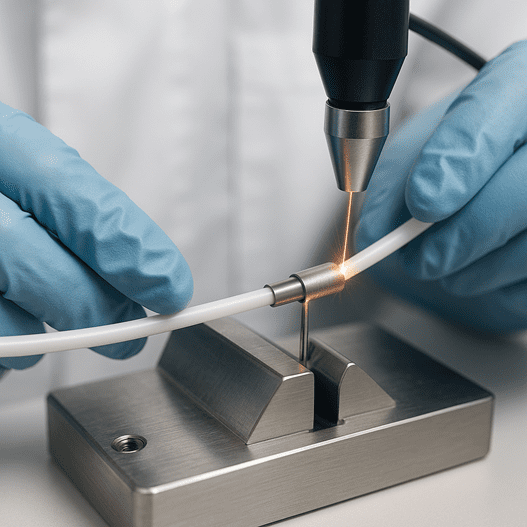
Laser welding uses a hair-thin, high-intensity light beam to join materials with micron-scale precision. By confining all the heat to a microscopic interaction zone, known as the heat-affected zone (HAZ), the process briefly melts the workpiece before it solidifies almost instantly. This tight thermal footprint (often under 50 µm) keeps nearby plastics, sensors, and coatings…
Read More